New Paper: Multiphoton 3D Microprinting of Protein Micropatterns with Spatially Controlled Heterogeneity – A Platform for Single Cell Matrix Niche Studies
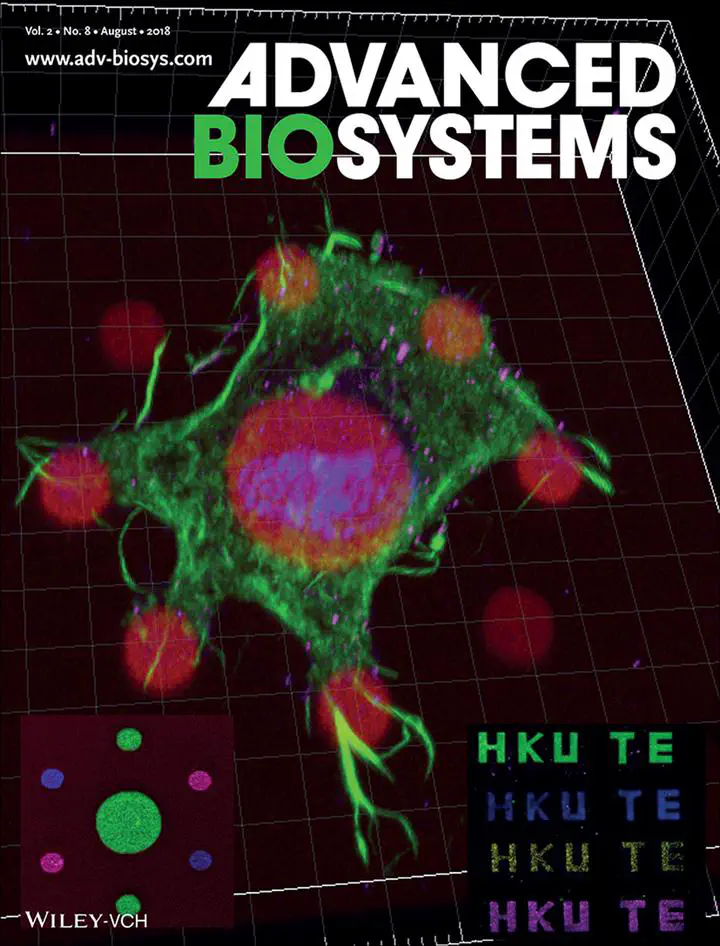
This study demonstrated that our multiphoton microprinting technology is able to precisely control the local density of the matrix niche factor, and more importantly, spatially control and decouple the mechanical and matrix niche factors. The results show that 1) the micropatterned matrix niche retains its bioactivities; 2) mesenchymal stem cells (MSCs) are able to sense the elastic modulus of the cell niche when the mechanical and matrix niche factors are decoupled; and 3) MSCs prefer to bind to and spread on fibronectin among other matrix proteins.
Check out the published paper here: https://doi.org/10.1002/adbi.201800053
Abstract
Reconstituting the biomimetic cell niche in vitro is important as it allows to determine how niche factors may affect cellular fate processes. Many biofabrication and micropatterning technologies are developed to achieve this goal. However, the critical outstanding challenges are the capabilities to spatially control the micropatterns and to decouple the different niche factors. Multiphoton microfabrication is an emerging technology with unique advantages. A multiphoton 3D microprinting platform has been previously established to create protein microstructures and micropatterns with sub-micrometer resolution and its ability to control the topological features, porosity, and mechanical properties of microprinted protein cell niche has been demonstrated. Here, it is demonstrated that the multiphoton microprinting technology is able to precisely control the local density of the matrix niche factor, and more importantly, spatially control and decouple the mechanical and matrix niche factors. The results show that 1) the micropatterned matrix niche retains its bioactivities; 2) mesenchymal stem cells (MSCs) are able to sense the elastic modulus of the cell niche when the mechanical and matrix niche factors are decoupled; and 3) MSCs prefer to bind to and spread on fibronectin among other matrix proteins. These results path the way to future development of a “programmable” cell niche platform.