New Paper: Mechanically induced formation and maturation of 3D-matrix adhesions (3DMAs) in human mesenchymal stem cells
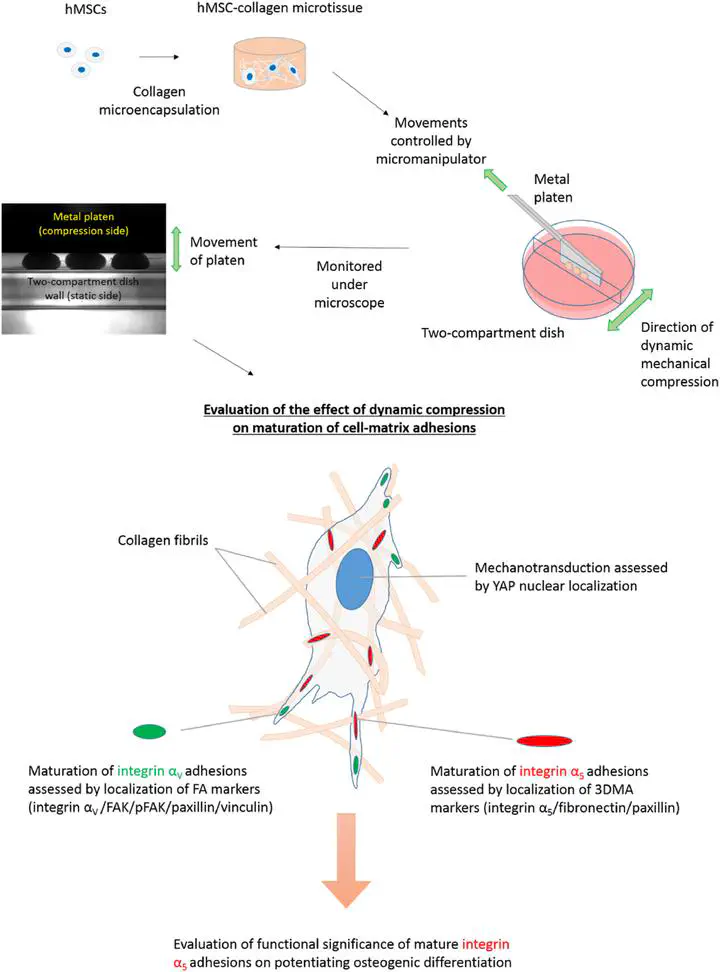
Our new paper explored the effects of compression loading in mesenchymal stem cells encapsulated in 3D collagen micro-tissues. Short-term dynamic compression results suggested crosstalk between integrin-based signalling and mechanosensing, while long-term compression loading induced maturation of 3D matrix adhesions (3DMAs) and potentiated hMSC osteogenesis.
Check out the published paper here: https://doi.org/10.1016/j.biomaterials.2020.120292
Abstract
Mechanical signal is important for regulating stem cell fate, but the molecular mechanisms involved are unclear. Cell-matrix adhesions are important molecular mechanosensors that their formation and maturation are force-dependent processes. However, most studies focused on the role of cell contractility or substrate stiffness in these processes. How external mechanical force stimulates the formation and maturation of cell-matrix adhesions is largely unknown. Here, by using human mesenchymal stem cells (hMSCs)-collagen microtissues as a 3D model, we found that upon short-term dynamic compression, integrin αV binding, focal adhesion formation, and subsequent FAK activation, are stimulated. This compression-stimulated FAK signaling also leads to YAP activation, suggesting crosstalk between integrin-based signaling and mechanosensing. More importantly, long-term compression induces maturation of α5-integrin based adhesions to form long, slender 3D-matrix adhesions (3DMAs), which are distinct from 2D focal adhesions in composition and morphology and previously found only in cell-derived matrices and native tissues. Mechanical preconditioning hMSCs with long-term compression loading induces the formation of mature integrin α5-dependent 3DMAs and potentiates their osteogenesis. Collectively, this work shows that active mechanical stimulation can modulate cell-matrix interactions significantly at the cell-material interfaces in a dynamic manner, and affects cell fate decisions, demonstrating the significance of loading-based functional tissue engineering.